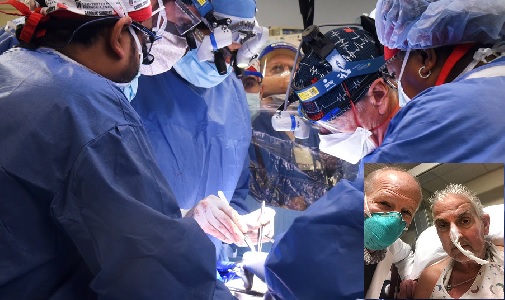
Washington (ISJ): Cardiac surgeons at the University of Maryland Medical Centre, successfully performed a historic first transplant of porcine heart into an adult end-stage heart patient.
The 57-year-old patient’s only available option for survival was to get a genetically modified pig heart. The historic surgery was conducted by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Centre (UMMC), together known as the University of Maryland Medicine.
The transplant demonstrated for the first time that a genetically-modified animal heart can function like a human heart without immediate rejection by the body. The patient, David Bennett, a Maryland resident, is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits. He had been deemed ineligible for a conventional heart transplant at UMMC as well as at several other leading transplant centres that reviewed his medical records.
“It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice,” said Bennett, a day before the surgery was conducted. He had been hospitalized and bedridden for the past few months. “I look forward to getting out of bed after I recover.”
U.S. Food and Drug Administration granted emergency authorization for the surgery on New Year’s Eve through its expanded access (compassionate use) provision. It is used when an experimental medical product, in this case the genetically-modified pig’s heart, is the only option available for a patient faced with a serious or life-threatening medical condition. The authorization to proceed was granted in the hope of saving the patient’s life.
“This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor human hearts available to meet the long list of potential recipients,” said Bartley P. Griffith, who transplanted the pig heart into the patient. “We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future.”
“This is the culmination of years of highly complicated research to hone this technique in animals with survival times that have reached beyond nine months. The FDA used our data and data on the experimental pig to authorize the transplant in an end-stage heart disease patient who had no other treatment options,” said Dr Muhammad M. Mohiuddin, Professor of Surgery at UMSOM. “The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.”
About 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients die each year before getting one, according to the federal government’s organdonor.gov. Transplanting animal organs, known as xenotransplantation, could potentially save thousands of lives but does carry a unique set of risks, including the possibility of triggering a dangerous immune response. These responses can trigger an immediate rejection of the organ with a potentially deadly outcome to the patient.
Xenotransplants were first tried in the 1980s, but were largely abandoned after the famous case of Stephanie Fae Beauclair (known as Baby Fae) at Loma Linda University in California. The infant, born with a fatal heart condition, received a baboon heart transplant and died within a month of the procedure due to the immune system’s rejection of the foreign heart. However, for many years, pig heart valves have been used successfully for replacing valves in humans.
Revivicor, a regenerative medicine company based in Blacksburg, Virginia, provided the genetically-modified pig to the xenotransplantation laboratory at UMSOM.
The physician-scientists also used a new drug along with conventional anti-rejection drugs, which are designed to suppress the immune system and prevent the body from rejecting the foreign organ. The new drug used is an experimental compound made by Kiniksa Pharmaceuticals.
Source: University of Maryland School of Medicine
Image courtesy: University of Maryland School of Medicine